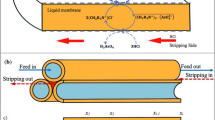
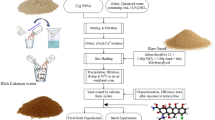
Abstract
Arsenic water contamination is a major issue worldwide, particularly in regions where groundwater is the primary source of drinking and irrigation water. Therefore, there is an urgent need of addressing this problem in an effective and environmentally friendly manner. So far, several conventional and emerging removal technologies have been implemented for arsenic removal from water, including coagulation, flocculation, adsorption and membrane-based separation approaches. Here we show the successful development of a new bio-inspired porous membrane that has been made selective for the removal of arsenic (As(V) as well as the more toxic and difficult to remove As(III)) present in arsenic-contaminated groundwaters. The arsenic removal efficiency of the membrane has been successfully assessed both in model solutions and in a real groundwater. Very importantly, owing to the high selectivity of the membrane towards arsenic, no substantial demineralization occurred with the real groundwater, which therefore became directly suitable for human consumption.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All relevant data generated and analysed during this study, which include experimental, spectroscopic, membrane characterization, analytic and computational data, are included in this article, its Supplementary Information and the Open Science Framework repository with the identifier https://osf.io/5xrab/.
References
Bundschuh, J. et al. Global arsenic dilemma and sustainability. J. Hazard. Mat. 436, 129197 (2022).
Shakoor, M. et al. Unraveling health risk and speciation of arsenic from groundwater in rural areas of Punjab, Pakistan. Int. J. Environ. Res. Public Health 12, 12371–12390 (2015).
Podgorski, J. & Berg, M. Global threat of arsenic in groundwater. Science 368, 845–850 (2020).
IARC working group on the evaluation of carcinogenic risks to humans, some drinking-water disinfectants and contaminants, including arsenic. IARC Monogr. Eval. Carcinog. Risks Humans 84, 1–477 (2004).
Guidelines for drinking-water quality: fourth edition incorporating the first addendum. World Health Organization https://doi.org/10.2105/AJPH.17.2.162-b (2017).
Algieri, C. et al. Arsenic removal from groundwater by membrane technology: advantages, disadvantages, and effect on human health. Groundwater Sustain. Dev. 19, 100815 (2022).
Dilpazeer, F. et al. A comprehensive review of the latest advancements in controlling arsenic contaminants in groundwater. Water 15, 478 (2023).
Alka, S. et al. Arsenic removal technologies and future trends: a mini review. J. Clean. Prod. 278, 123805 (2021).
Ge, Q., Lau, C. H. & Liu, M. A novel multi-charged draw solute that removes organic arsenicals from water in a hybrid membrane process. Environ. Sci. Technol. 52, 3812–3819 (2018).
Nurchi, V. M. et al. Arsenic toxicity: molecular targets and therapeutic agents. Biomolecules 10, 235 (2020).
Zhang, S.-Y. et al. Poly(ionic liquid) composites. Chem. Soc. Rev. 49, 1726–1755 (2020).
Smedley, P. & Kinniburgh, D. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 17, 517–568 (2002).
Shen, S., Li, X.-F., Cullen, W. R., Weinfeld, M. & Le, X. C. Arsenic binding to proteins. Chem. Rev. 113, 7769–7792 (2013).
Sing, K. S. W. et al. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 57, 603–619 (1985).
Jain, A. & Loeppert, R. H. Effect of competing anions on the adsorption of arsenate and arsenite by ferrihydrite. J. Environ. Qual. 29, 1422–1430 (2000).
Davis, S. A. & Misra, M. Transport model for the adsorption of oxyanions of selenium (IV) and arsenic (V) from water onto lanthanum- and aluminum-based oxides. J. Colloid Interface Sci. 188, 340–350 (1997).
Biswas, A. et al. Role of competing ions in the mobilization of arsenic in groundwater of Bengal Basin: insight from surface complexation modeling. Water Res. 55, 30–39 (2014).
Youngran, J., Fan, M., Van Leeuwen, J. & Belczyk, J. F. Effect of competing solutes on arsenic(V) adsorption using iron and aluminum oxides. J. Environ. Sci. 19, 910–919 (2007).
Nicomel, N., Leus, K., Folens, K., Van Der Voort, P. & Laing, G. D. Technologies for arsenic removal from water: current status and future perspectives. Int. J. Environ. Res. Public Health 13, 62 (2015).
Singh, R., Singh, S., Parihar, P., Singh, V. P. & Prasad, S. M. Arsenic contamination, consequences and remediation techniques: a review. Ecotoxicol. Environ. Saf. 112, 247–270 (2015).
Giles, D. E., Mohapatra, M., Issa, T. B., Anand, S. & Singh, P. Iron and aluminium based adsorption strategies for removing arsenic from water. J. Environ. Manage. 92, 3011–3022 (2011).
Roy, P. K., Choudhury, M. R. & Ali, M. A. As (III) and As (V) Adsorption on magnetite nanoparticles: adsorption isotherms, effect of pH and phosphate, and adsorption kinetics. Int. J. Chem. Environ. Eng. 4, 55–63 (2013).
Pena, M. E., Korfiatis, G. P., Patel, M., Lippincott, L. & Meng, X. Adsorption of As(V) and As(III) by nanocrystalline titanium dioxide. Water Res. 39, 2327–2337 (2005).
Tang, W., Li, Q., Gao, S. & Shang, J. K. Arsenic (III,V) removal from aqueous solution by ultrafine α-Fe2O3 nanoparticles synthesized from solvent thermal method. J. Hazard. Mater. 192, 131–138 (2011).
Petosa, A. R., Jaisi, D. P., Quevedo, I. R., Elimelech, M. & Tufenkji, N. Aggregation and deposition of engineered nanomaterials in aquatic environments: role of physicochemical interactions. Environ. Sci. Technol. 44, 6532–6549 (2010).
Siddique, T., Dutta, N. K. & Choudhury, N. R. Nanofiltration for arsenic removal: challenges, recent developments, and perspectives. Nanomaterials 10, 1323 (2020).
Fang, J. & Deng, B. Rejection and modeling of arsenate by nanofiltration: contributions of convection, diffusion and electromigration to arsenic transport. J. Membr. Sci. 453, 42–51 (2014).
Sato, Y., Kang, M., Kamei, T. & Magara, Y. Performance of nanofiltration for arsenic removal. Water Res. 36, 3371–3377 (2002).
Criscuoli, A. & Figoli, A. Pressure-driven and thermally-driven membrane operations for the treatment of arsenic-contaminated waters: a comparison. J. Hazard. Mater. 370, 147–155 (2019).
Qasim, M., Badrelzaman, M., Darwish, N. N., Darwish, N. A. & Hilal, N. Reverse osmosis desalination: a state-of-the-art review. Desalination 459, 59–104 (2019).
Clancy, T. M., Hayes, K. F. & Raskin, L. Arsenic waste management: a critical review of testing and disposal of arsenic-bearing solid wastes generated during arsenic removal from drinking water. Environ. Sci. Technol. 47, 10799–10812 (2013).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys. 132, 154104 (2010).
Frisch, M. J. et al. Gaussian 16, Revision D.01 (Gaussian, Inc., 2016).
Tomasi, J., Mennucci, B. & Cancès, E. The IEF version of the PCM solvation method: an overview of a new method addressed to study molecular solutes at the QM ab initio level. J. Mol. Struct. 464, 211–226 (1999).
Doherty, S. et al. Highly efficient aqueous phase chemoselective hydrogenation of α,β-unsaturated aldehydes catalysed by phosphine-decorated polymer immobilized IL-stabilized PdNPs. Green Chem. 19, 1635–1641 (2017).
Lin, X., Godeau, G. & Grinstaff, M. W. A reversible supramolecular assembly containing ionic interactions and disulfide linkages. New J. Chem. 38, 5186–5189 (2014).
Simone, S. et al. Preparation and characterization of polymeric-hybrid PES/TiO2 hollow fiber membranes for potential applications in water treatment. Fibers 5, 14 (2017).
Figoli, A. et al. Innovative hydrophobic coating of perfluoropolyether (PFPE) on commercial hydrophilic membranes for DCMD application. J. Membr. Sci. 522, 192–201 (2017).
Galiano, F. et al. A step forward to a more efficient wastewater treatment by membrane surface modification via polymerizable bicontinuous microemulsion. J. Membr. Sci. 482, 103–114 (2015).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Newville, M. EXAFS analysis using FEFF and FEFFIT. J. Synchrotron Radiat. 8, 96–100 (2001).
Apollaro, C., Fuoco, I., Brozzo, G. & De Rosa, R. Release and fate of Cr(VI) in the ophiolitic aquifers of Italy: the role of Fe(III) as a potential oxidant of Cr(III) supported by reaction path modelling. Sci. Total Environ. 660, 1459–1471 (2019).
Figoli, A. et al. Arsenic-contaminated groundwaters remediation by nanofiltration. Sep. Purif. Technol. 238, 116461 (2020).
Meng, X., Korfiatis, G. P., Jing, C. & Christodoulatos, C. Redox transformations of arsenic and iron in water treatment sludge during aging and TCLP extraction. Environ. Sci. Technol. 35, 3476–3481 (2001).
Wolery T. W. & Jarek, R. L. Software User’s Manual. EQ3/6, Version 8.0 (Sandia National Laboratories, 2003).
Marini, L. & Accornero, M. Prediction of the thermodynamic properties of metal–arsenate and metal–arsenite aqueous complexes to high temperatures and pressures and some geological consequences. Environ. Geol. 52, 1343–1363 (2007).
Acknowledgements
The research leading to these results received funding from the Regione Calabria (Italy), POR Project AsSE, grant no. J28I17000030006 ‘Arsenic separation from waters by membrane processes’ (A.F., C.A. and B.G.). The authors thank M. Frappa from Institute on Membrane Technology, National Research Council, for his assistance in membrane preparation. We dedicate this paper to the memory of Cinzia Chiappe, a truly exceptional scholar and researcher.
Author information
Authors and Affiliations
Contributions
F.G., R.M., C.S.P., J.B., J.R., C.C., A.F. and B.G. conceptualized and designed the study. F.G. and A.F. prepared and characterized the membranes. R.M. and L.G. prepared and characterized the polymerizable ionic liquids. C.S.P. performed the theoretical calculations. F.G. conducted the As extraction tests. S.-L.W. carried out the EXAFS synchrotron studies. C.A. collected the natural groundwater and performed the chemical analyses. F.P. conducted the XPS studies. F.G. and B.G. wrote the original draft of the manuscript, with the contributions of all other authors. F.G., A.F. and B.G. contributed to the writing of all subsequent versions of the manuscript. B.G. supervised, convened and coordinated the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Water thanks Cher Hon Lau and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Details on theoretical calculations on the interactions between PIL-S-M and As(V) or As(III), Supplementary Figs. 1–12 and Tables 1 and 2, and details on cross-flow filtration tests.
Source data
Source Data Fig. 2
Statistical source data for Fig. 2a,b.
Source Data Fig. 4
Statistical source data for Fig. 4a,c.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Galiano, F., Mancuso, R., Guazzelli, L. et al. Arsenic water decontamination by a bioinspired As-sequestering porous membrane. Nat Water 2, 350–359 (2024). https://doi.org/10.1038/s44221-024-00220-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44221-024-00220-x
This article is cited by
-
A bio-inspired membrane for arsenic removal
Nature Water (2024)