Abstract
High Latitude fjords can serve as sediment trap, bearing different type of proxies, from geochemical to micropaleontological ones, making them exceptional tools for paleoenvironmental reconstruction. However, some unconventional proxies can be present and can be used to depict a comprehensive and exhaustive interpretation of past changes. Here, studying a sediment core in Edisto Inlet (Ross Sea, Antarctica) we used irregular echinoid spines and ophiuroids (Ophionotus victoriae) ossicles to trace environmental changes throughout the last 3.6 kyrs BP. Irregular echinoids can serve as proxy for the organic matter content, while O. victoriae ossicles can be used as proxy for steady sea-ice cycle along with organic deposition events. O. victoriae release a high number of ossicles, making estimation about the population quite challenging; still, presence data, can be easily collected. By applying Generative Additive Models to the stratigraphical distribution of these data, we detected an environmental phase that was previously unnoticed by other traditional proxies: the Ophiuroid Optimum (2–1.5 kyrs BP). In conclusion, here we demonstrate how echinoderm presence can be used as a valuable source of information, while proving the potential of modelling binary data to detect long-term trend in Holocene stratigraphical records.
Similar content being viewed by others
Introduction
Fjords are one of the most important transitional environments of the high latitudes. Recently they have been identified as carbon cycle hotspots making them an important factor in Earth’s climate system, being the marine environment with the highest carbon burial rate per unit area 1,2. However, most of the studied fjords are in the northern Hemisphere, especially in the Arctic, whilst the southern counterpart remains less studied with the Antarctic peninsula being the only exception3. In their role as sedimentation traps, fjords have been extensively used in paleoenvironmental reconstruction, especially to disentangle abrupt changes in the environment over the Holocene. This has been of great importance in the Antarctic Peninsula to recognize glacial advance or retreat, meltwater pulses, changes in the hydrographic conditions and in sedimentation regime3,4,5,6,7. A plethora of methods have been used to investigate these aspects: from micropaleontological analysis on foraminifera and diatoms8, to geochemical proxies and biomarkers9.
In addition, these highly dynamic systems have been studied for their modern ecosystem characteristic. Bae et al.10 showed that different species of diatoms, located in different parts of the Marian coves, can build up different macrofauna communities. Lagger et al.11, studying Potter Cove in 2010, found that ascidians and bryozoans constitute more than 90% of the total benthic community of a newly ice-free area.
Despite the increased attention towards the environmental evolution of fjords, little attention has been paid to the use of macrofauna component in paleoenvironmental reconstruction regardless of their importance as a tool to give insights on community and environmental dynamics12,13.
Whitin this context, our study focuses on echinoderm remains in the Edisto Inlet, a small fjord located in the Ross Sea characterized by a seasonal sea-ice cover to assess if macrofaunal component can be used to depict a more comprehensive view of the environmental evolution of this area. The fjord has been previously studied for paleoenvironmental dynamics, seafloor characteristics and tephra content14,15,16,17,18. The bottom of the Inlet is covered by a 110 m thick expanded laminated Holocene sedimentary sequence, reflecting different diatom communities through distinct lamina colours17,18. Although echinoderm remains (ophiuroids and echinoids) were previously used to develop the age-depth model of two cores inside the Inlet, no analysis on their stratigraphical distribution was conducted18,19.
Echinoderms are one of the most important benthic groups in Antarctic waters, constituting most of the total benthic biomass20,21,22. Moreover, this group plays a fundamental role in the community structure making it a valuable source of information for ecosystem dynamics23,24,25,26. Upon decay, echinoderms release a high number of ossicles (several thousand per individual in case of the ophiuroids). Thanks to recent advances in understanding of lateral arm plate (LAP) morphology of ophiuroids, aligned with genomic studies, isolated ophiuroid ossicles have been identified as powerful tool for micropaleontological analyses27,28,29. The goal of this study is to provide a case study for the use of echinoderm ossicles as an ecological and paleoenvironmental proxy and thereby unlock the micropaleontological potential of echinoderms as key macrofaunal component.
Study area
Edisto Inlet, situated in the northwestern part of the Ross Sea, is an elongated and narrow fjord with an average depth of 500 m and a minimum depth of 100 m at the fjord mouth14,17 (Fig. 1). The fjord has four different glacial inputs: the Edisto glacier, the Manhaul glacier, and the Arneb glacier and a little unnamed glacier near the mouth(Fig. 1). Geomorphological evidence, combined with geochemical and micropaleontological analyses, was used previously to study the environmental evolution of the Inlet over the last 11 kyrs BP15,17,18.
From 11 kyrs BP, the glaciers started to retreat, leading to open water conditions, along with the reinvigoration of the circulation. The period from 9 to 2.6 kyrs BP witnessed a deglaciation phase with the establishment of a seasonal sea-ice regime17. Subsequently, from 2.6 to 0.7 kyrs BP, geochemical and foraminifera analysis suggested the presence of a seasonal sea-ice cover, and around 1.48 kyrs BP, conspicuous glacial meltwater flow attributed to the retreat of three in-situ glaciers was linked with the onset of the Medieval Climate Anomaly (MCA)15,18. Lastly, spanning from 0.7 kyrs BP to the present day, after the onset of the Little Ice Age (LIA), a prolonged period of sea-ice cover has been suggested by the sudden decrease of the sedimentation rate by one order of magnitude, probably due to persisting presence of the sea-ice during the thawing season15,16,18.
Materials and methods
Core TR17-08
The marine sediment core TR17-08 (14.6 m long) was retrieved in January 2017 at the entrance of the fjord at a depth of 462 m below sea level (Fig. 1). The core consists of diatomaceous ooze and shows a lamination defined by the alternation of olive-green to brownish lamina (dark), and white ones (light). In Edisto, lamina colour reflects diatom assemblages: dark laminae are dominated by Fragilariopsis curta, F. obliquecostata and have a low biovolume content of Corethoron pennatum, indicative of a first sea-ice break-up during the early austral summer, whilst light laminae are mainly composed by C. pennatum and are deposited during the later part of the summer, when oligotrophic conditions are present18 (Fig. S1). The age depth-model was constructed using 10 radiocarbon dates and 1 tephra layer associated with the Mount Rittmann eruption15,16 (Fig. S2). The core covers a period of 3.6 kyrs BP and, around 0.7 kyrs BP, in concomitance with the Mount Rittman tephra, the sedimentation rate changes from an average of 0.47 cm yr−1 to an average of 0.07 cm yr−116,18 (Fig. S2).
Micropaleontological samples
Samples with 1 cm thickness were taken every 10 cm from core TR17-08, resulting in a total of 152 samples. Samples were washed with a 63 µm sieve and dried overnight at 40 °C. Ice Rafted Debris (IRD) content was determined by counting sharp clastic remains in the > 1 mm fraction. Foraminifera and echinoderm remains in the > 150 µm fraction were picked exhaustively. Since autotomy and arm regeneration can exaggerate the number of ossicles released into the environment by ophiuroid individuals30, we used the distribution of ossicles to evaluate the presence/absence of the echinoderm taxa. Benthic Foraminifera Accumulation Rate (BFAR, specimens cm−2 yr−1), Planktic Foraminifera Accumulation Rate (PFAR, specimens cm−2 yr−1) and IRD fluxes (counts·cm−2 yr−1) were measured as described in Herguera and Berger31. Foraminifera were recognised at the species level (for an extensive list of the species see table S1) using the taxonomic table from32,33,34,35,36. Ophiuroid remains were analysed using Scanning Electron Microscopy (SEM) to evaluate the diagnostic features of the LAPs29,37. Echinoid spines were collected and recognised at the functional level.
Statistical analysis
Presence/Absence data are difficult to interpret due to their binomial nature. Generative Additive Models (GAM) were used to model the probability of occurrence over time, by utilizing the method to detect trends in temporal series described by Simpson38. GAM are homologous to the LOESS curve but rely on fewer assumptions, giving these models an advantage since they can detect significant trends in temporal series 38,39. The age (in yrs BP) of the layer was used as a predictor of the model. GAM were applied to both echinoid and ophiuroid distribution. Statistical analyses were performed in the software RStudio40 (v4.3.1). GAM were calculated using the mgcv package41. The fit criterion used was the restricted maximum likelihood (REML, Simpson, 2018). For the stratigraphical analysis, a stratigraphically constrained analysis with Euclidean distance (CONISS) was computed using BFAR, PFAR, IRD, Margalef (M), Eveness (J) and the probability of occurrence of the echinoids (P(E)) and ophiuroids (P(O)). We used the package tidypaleo for the CONISS, which uses a broken stick approach to define significant clusters42. Foraminifera diversity indexes, M and J, were calculated using the software PAST43 (v4.14). M index is calculated using the formula \(M = \left( {S - 1} \right)/ln(n\)), where S is the number of species and n is the total number of individuals. J is calculated using the formula \(J = e^{H} /S\), where H is the Shannon Index, corresponding to \(H = - \mathop \sum \limits_{i} \frac{{n_{i} }}{n}\ln \frac{{n_{i} }}{n}\).
Results and discussion
Ophiuroids and echinoids
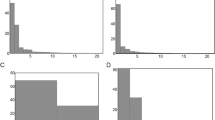
In our record echinoids were more common throughout (104/152 samples, 69%) than ophiuroids (27/152 samples, 18%) but were consistently absent from 1 to 0.7 kyrs BP (Fig. 2). Ophiuroid presence is more frequent during the period that goes from the 3.6 to 1 kyrs BP. GAM applied to the distribution shows significant trends (p < 0.05), increasing their values as the density of the presence points increases (Fig. 2; Table S2). The P(E) increases from 3.6 to 1.5 kyrs BP, while it tends to decrease after 1.5 kyrs, where the echinoderms are absent (Fig. 2a). Ophiuroids show a different trend: the P(O) increases steadily from the bottom and peaks around 2 kyrs BP, in concomitance with the interval with most presence points (Fig. 2b). Although the peak of the P(O) is low (< 0.5), we argue that these low values arise due to the low number of presence points of the ophiuroid distribution (Fig. 2b). Despite the low probability values, the P(O) still gives important clues on the long-term trend, as it can define the frequent presence of ophiuroids, as seen by the bulging of the curve in the interval 2–1.5 kyrs BP (Fig. 2b).
GAM model of the echinoderm’s distribution. (a) GAM model of the irregular echinoids; (b) GAM model of the ophiuroid. P(E) refers to the probability of occurrence of the echinoids, while P(O) is the probability of occurrence of the ophiuroids. Trend line is displayed as black line, and the confidence band (95%) is indicated by the light blue ribbon. Black points represent the presence (1)/ absence (0) distribution along the core.
This application shows the strength of utilizing GAM models even in binomial distribution, unlocking possibilities on the use of this type of models to understand long-term macrofauna dynamics just by a presence/absence matrix derived from a temporal series.
Micromorphological analysis of the LAPs retrieved from our samples allowed us to recognize the presence of the species Ophionotus victoriae Bell, 190229,37,44. Since there were no other types of LAP throughout the core, we assume that all other ophiuroid ossicles belonged to the same species (Figs. 3 and 4).
Skeletal plates of Ophionotus victoriae (scale bars = 500 µm): (A, B) vertebrae, proximal and distal views; (C, D) broken Dental plates, external and internal view; (E, F) proximal lateral arm plate, external and internal views; (G) adradial genital plate; H-I. Dorsal arm plate, internal and external views; (J, K) distal lateral arm plate, external and internal views.
Ophionotus victoriae is a well-known and widely distributed species across Antarctica, living at almost every depth and within different types of environments20. Like other ophiuroids in Antarctica, O. victoriae is an opportunistic species with a high diet plasticity, even showing cannibalistic behaviour in high-density populations 45. Cannibalistic behaviour is typically found among organisms that live in areas affected by strong seasonal fluctuations, like fjords or high-latitude enclosed basins, generally characterized by a seasonal cycle of the sea-ice cover26,45,46. In Deception Island, O. victoriae spatial distribution relates to the sedimentation regime and a low ice-related disturbance21,47. In addition, O. victoriae has a peculiar reproductive biology involving synchronous annual spawning in November–December, long oocyte development periods and, the dependence upon the previous year sedimentation event of the reproductive effort48. Considering these premises, we hypothesize that the presence of O. victoriae could be a valuable proxy for low interannual variability of the environmental cycle of the study area that corresponds to a seasonal sea-ice cycle along with constant organic deposition events. Given the crucial role of O. victoriae in the bentho-pelagic coupling of the community, its presence could also be used as a proxy to indicate a mature benthic community, implying that an energy flux was present from the primary producers (e.g. diatoms) to the secondary consumers24.
Since the skeleton of O. victoriae is made of high-Mg calcite, the ossicles can be prone to dissolution after decay, with a complete disappearance estimated to occur within 6–105 years49. However, in high sedimentation regimes, like the Edisto Inlet, rapid burial could have removed the ossicles from corrosive water masses enabling their preservation50. Considering the similar chemical composition of O. victoriae ossicles and echinoid spines and considering the coupled presence of both along the core, we infer that there was no taphonomical filter on the distribution of the echinoderm remains51.
Echinoid spines were identified as belonging to irregular echinoids (infraclass Irregularia, Latreille 1825, Fig. S3) due to their morphology, although the material available precluded a species-level identification52,53. Irregular echinoids are bottom dwellers feeding on organic matter on and within the seafloor54. This type of ecology hints at a possible use as proxy for organic matter content on the seafloor.
Paleoenvironmental reconstruction over the Late Holocene of Edisto Inlet
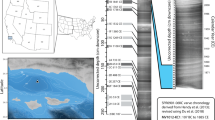
To test whether the echinoderms could be used as a proxy for past environmental conditions, we compared P(E) and P(O) with other proxies derived from the core TR17-08 and from biogeochemical proxies derived from a nearby core inside the Inlet, HLF17-01 (Figs. 5 and 6). BFAR and PFAR have been used extensively as proxy for the paleo productivity31,55. Also, IRD fluxes have been used to reconstruct the location of the polar front, iceberg discharging events and the extension of the sea-ice cover at both poles56,57,58,59. In Barilari Bay (Antarctic Peninsula), a fjord with similar environmental features as Edisto, high abundances of IRD suggests the onset of a discharging event guided by calving of an iceberg in a marine terminating glacier, and a decrease in its content marks the onset of seasonally open marine conditions59.
Comparison between the BFAR, PFAR, IRD, J, D, P(O) and P(E) over 3.6 kyrs BP from core TR17-08. CONISS cladogram is also displayed. The dashed lines represent the CONISS cluster division. Note the different scale on every graph. BFAR, PFAR and IRD are calculated in counts·cm−2·yr−1. The grey ribbon in the P(O) and P(E) graph represents the 95% confidence interval.
IPSO25 values over the last 2.6 kyrs BP in the core HLF17-01 (Tesi et al.18). The blue dots indicate summer sea-ice cover while red dots indicate free-sea ice conditions during the summer, the red shaded area highlights the period where P(E) and P(O) reaches the maximum. The y-axis is in logarithm scale.
Changes in J and M have been related to change in the benthic environment as well as the presence of stressful periods or events60,61,62. In the Arctic, changes in the population of calcareous foraminiferal fauna diversity and densities are related to changes in the macrofauna component, paralleling the response of the macrofauna diversity62. In our study, the diversity indexes calculated on the foraminiferal content are used as a proxy for the patchiness of the environmental conditions. Foraminiferal fauna composition depends upon the physical and chemical characteristics of the environment. Thus, an increase in the number of species could be evidence of an increase in the number of habitats or ecological niches61. On the other hand, J is used to evaluate how well the species are partitioned in a community, with lower values indicating that few species are dominating the community63. This can be interpreted as the prominent presence of the ecological characteristics of the dominant group. In other words, if J decreases and M increases, we can suppose that the decrease in the equitability (increase in the dominance of a species or a group) and the contemporary increase in the number of species, reflects the prominent presence of an environment type (indicated by the low values of J), while new rare species are added (indicated by M). In turn, this implies the steadiness of the ecological condition, since one species (or group) is dominating the assemblages, while not taking into consideration the environmental condition itself.
Considering these premises, the values of P(O) and P(E) should be higher in concomitance with relatively high primary productivity (High BFAR and PFAR), seasonal sea-ice cycle (low content of IRD), and an increase in the environmental steadiness (low J, high M).
The CONISS analysis divided our record into 4 clusters (Fig. 5, dashed lines). Starting from the oldest, the first cluster (Fig. 5, yellow band) goes from 3.6 to 2.5 kyrs BP. Within this interval the BFAR and PFAR show similar patterns, indicating a period with explosive increases of the primary productivity55. IRD fluxes are relatively high with respect to the subsequent zone (Fig. 5, red band), while J and M do not show any trend. This can be interpreted as a climatic phase where primary productivity was high but inconsistent from year to year, indicated by the peaks of IRD (Fig. 5, yellow band). The same conclusion can be inferred by the peaks in the BFAR and PFAR content and by the variability of J59,64 (Fig. 5, yellow band). The increase in the P(E) and P(O) corroborates this view. The increase in P(E) suggests an increase in the organic matter content on the seafloor probably derived from superficial algal blooms along with the increase in P(O) that can be interpreted as a gradual increase in the stability of the seasonal sea-ice cycle.
The subsequent interval (2.5–1.4 kyrs BP, Fig. 5 red band) is characterized by low values of BFAR, PFAR and IRD. During this time, J decreases in the middle part along with an increase in M. The low values of BFAR and PFAR can be interpreted as a decrease in primary productivity alongside a decrease in the variability of the input of nutrients, indicated by the lower values of the peaks (Fig. 5, red band). The IRD content is lower than the previous interval (Fig. 5, yellow band) and shows less variability, indicating the presence of a seasonal sea-ice cover59. During this period, P(O) and P(E) peaks at around 2–1.8 kyrs BP, validating our hypothesis that ophiuroid presence/absence can be used as a paleoenvironmental proxy, since the premises stated previously are met: low IRD content, relatively high BFAR and PFAR, and a decrease in J along with an increase in M.
In addition, this interval can be compared with the geochemical data from core HLF17-01 and with the foraminifera analysis from core TR17-0815,18. In the study of Tesi et al.18, IPSO25 index (Ice Proxy for the Southern Ocean with 25 highly branched isoprenoids65) have been used to track absence of sea-ice cover during the austral summer, revealing a prominent ice-free summer season from 2.6 to 0.7 kyrs BP (Fig. 6). However, the interval with the average lower values and with the most frequent under-the-threshold values of the IPSO25 spans the interval from 2 to 1.5 kyrs BP (Fig. 6). This period also corresponds to the peak in both P(E) and P(O) values, corroborating the hypothesis on the use of Ophionotus victoriae as a proxy for seasonal sea-ice cycle (Fig. 6). Foraminiferal analysis also supported the view of a prominent seasonal phase during this period15. By combining the results from the geochemical and the foraminiferal analyses with our echinoderm distribution we can identify a time interval that is characterized by an interannually stable sea-ice cycle with conspicuous organic sedimentation events, along with an energy flux that goes from primary producers (diatoms) to secondary consumers (ophiuroids and echinoids), implying the presence of a developed benthic community. We call this period Ophiuroid Optimum due to the relationship between the ophiuroid presence and the IPSO25 value (Figs. 5 and 6).
From 1.4 to 0.7 kyrs BP a transitional phase took place (Fig. 5, purple and blue bands). BFAR and PFAR drop to near 0 values, while IRD content suddenly increases at 1.2 kyrs BP. J increases, while M decreases in concomitance with P(E) and P(O) over the same period (Fig. 5, purple band). The period after 0.7 kyrs BP (Fig. 5, white band), experiences a drop in the sedimentation rate from an average value of 0.49 to 0.07 cm yr−1 and we decide to divide it from the previous cluster manually, since it yields less resolution, suggesting a closed environment with a sea-ice cover that does not thaw during the summer, or experience very incipient opening15,18,19. Foraminiferal analysis over 1.4–0.7 kyrs BP suggests the presence of a conspicuous meltwater flow, probably derived from the retreat of in-situ glaciers with the onset of the MCA, a warm phase recognised in the Northern Hemisphere as well as in the Antarctic Peninsula and in the Victoria Land Coast 15,66. The transitional phase has been interpreted as a deterioration of the seasonal sea-ice cycle, with an increase in the residence time of water masses inside the fjord15. The increase in J in concomitance with a decrease in M of the foraminiferal community also suggests a transitional state, where species vanish from the community, while the remaining ones thrive.
In addition, from another nearby core, BAY-0520 (Fig. 1), the increase in the Fragilariopsis curta content, a sea-ice indicator diatom, over the same period, is coherent with our interpretation67. Thus, our results suggest a period of less stable seasonality of the sea-ice cover, with a prolonged season of the winter cover, as suggested by the low BFAR and PFAR values. IRDs have the highest values over this period, and the steady decreases of P(E) and P(O), along with the increase in J and the decrease in M, corroborate the hypothesis of a reduction in the seasonality as well as the decrease in productivity, culminating around 0.7 kyrs BP15,18. In addition, the absence of both echinoids and ophiuroids (Fig. 2) suggests the absence of an energy flux that goes from the primary producers to the secondary consumers, reflected in the absence of a mature benthic macrofaunal community.
The period from 0.7 kyrs BP to recent (Fig. 5, white band) has been associated with the onset of the LIA, a northern hemisphere cooling period that has also been identified in Antarctica ice cores by a sudden decrease of 2 °C in the reconstructed air temperature68,69. Over this period, the sedimentation rate in Edisto Inlet is stable but with low value, suggesting the presence of a prolonged period of sea-ice cover with low-to-no productivity15,18. Results from P(E) and P(O) are difficult to interpret due to the low number of samples with respect to the previous zones, and the increase in their value and in the amplitude of the confidence interval could be derived from the low resolution of this period compared to other ones (Fig. 5).
Conclusion
In this study we evaluated the use of microfossils of macrofaunal organisms as paleoenvironmental proxies in high sedimentation settings by studying the marine sediment core TR17-08 in the Edisto Inlet, Ross Sea (Antarctica). In the record, spanning over 3.6 kyrs BP, we were able to successfully identify the presence of an ophiuroid species, Ophionotus victoriae, by analysing lateral arm plate (LAP) morphology along with the presence of irregular echinoid spines. To detect significant trends in the stratigraphical distribution of the latter, a presence/absence matrix was constructed. Generative Additive Models (GAM) were used to convert a binomial distribution into a continuous distribution, serving as a new way of analysing macrofaunal presence in paleoenvironmental studies. By comparing presence of echinoids (P(E)) and ophiuroids (P(O)), respectively, with other biogeochemical and micropaleontological proxies, we were able to demonstrate the use of O. victoriae as a proxy for the interannual steadiness of the seasonal sea-ice cycle and the organic sedimentation events, as well as a functional bentho-pelagic coupling. Although the irregular echinoid spines could not be identified in detail, their presence can be used to infer the existence of organic matter at the seafloor.
By utilizing geochemical, micropaleontological and macrofaunal proxies we were able to identify four different phases, in accordance with previous studies and a new seasonally stable phase called “Ophiuroid Optimum”15,18.
This study demonstrates that macrofaunal components can be used in micropaleontological studies and in multiproxy approaches to reconstruct paleoenvironmental settings, opening new ways to describe and interpret past climatic and environmental changes.
Data availability
The data used to produce the results in this study are available in the supplementary material of the article.
References
Bianchi, T. S. et al. Fjords as aquatic critical zones (ACZs). Earth Sci. Rev. 203, 103145 (2020).
Smith, R. W., Bianchi, T. S., Allison, M., Savage, C. & Galy, V. High rates of organic carbon burial in fjord sediments globally. Nat. Geosci. 8, 450–453 (2015).
Cottier, F. R. et al. Arctic fjords: A review of the oceanographic environment and dominant physical processes. Geol. Soc. Spec. Publ. 344, 35–50 (2010).
Allen, C. S., Oakes-Fretwell, L., Anderson, J. B. & Hodgson, D. A. A record of Holocene glacial and oceanographic variability in Neny Fjord, Antarctic Peninsula. Holocene 20, 551–564 (2010).
Kim, S. et al. Holocene paleoceanography of Bigo Bay, west Antarctic Peninsula: Connections between surface water productivity and nutrient utilization and its implication for surface-deep water mass exchange. Quat. Sci. Rev. 192, 59–70 (2018).
Domack, E. W. & McClennen, C. E. Accumulation of glacial marine sediments in fjords of the Antarctic Peninsula and their use as Late Holocene paleoenvironmental indicators. Found. Ecol. Res. West Antarctic Peninsula 70, 135–154 (1996).
García, M. et al. Geomorphic and shallow-acoustic investigation of an Antarctic Peninsula fjord system using high-resolution ROV and shipboard geophysical observations: Ice dynamics and behaviour since the Last Glacial Maximum. Quat. Sci. Rev. 153, 122–138 (2016).
Knudsen, K. L., Stabell, B., Seidenkrantz, M.-S., EirÍKsson, J. Ó. N. & Blake, W. Deglacial and Holocene conditions in northernmost Baffin Bay: Sediments, foraminifera, diatoms and stable isotopes. Boreas 37, 346–376 (2008).
Barbara, L., Crosta, X., Schmidt, S. & Massé, G. Diatoms and biomarkers evidence for major changes in sea ice conditions prior the instrumental period in Antarctic Peninsula. Quat. Sci. Rev. 79, 99–110 (2013).
Bae, H. et al. Shift in polar benthic community structure in a fast retreating glacial area of Marian Cove, West Antarctica. Sci. Rep. 11, 241 (2021).
Lagger, C., Servetto, N., Torre, L. & Sahade, R. Benthic colonization in newly ice-free soft-bottom areas in an Antarctic fjord. PLoS One 12, e0186756 (2017).
Moffitt, S. E., Hill, T. M., Roopnarine, P. D. & Kennett, J. P. Response of seafloor ecosystems to abrupt global climate change. Proc. Natl. Acad. Sci. U. S. A. 112, 4684–4689 (2015).
Singh, A., O’Regan, M., Coxall, H. K., Forwick, M. & Löwemark, L. Exploring late Pleistocene bioturbation on Yermak Plateau to assess sea-ice conditions and primary productivity through the Ethological Ichno Quotient. Sci. Rep. 13, 17416 (2023).
Finocchiaro, F. et al. Record of the early Holocene warming in a laminated sediment core from Cape Hallett Bay (Northern Victoria Land, Antarctica). Glob. Planet Change 45, 193–206 (2005).
Galli, G. et al. Paleoenvironmental changes related to the variations of the sea-ice cover during the Late Holocene in an Antarctic fjord (Edisto Inlet, Ross Sea) inferred by foraminiferal association. J. Micropalaeontol. 42, 95–115 (2023).
Di Roberto, A. et al. Cryptotephras in the marine sediment record of the Edisto Inlet, Ross Sea: Implications for the volcanology and tephrochronology of northern Victoria Land, Antarctica. Quat. Sci. Adv. 2023, 10 (2023).
Battaglia, F. et al. The discovery of the southernmost ultra-high-resolution Holocene paleoclimate sedimentary record in Antarctica. Mar. Geol. 467, 107189 (2024).
Tesi, T. et al. Resolving sea ice dynamics in the north-western Ross Sea during the last 2.6 ka: From seasonal to millennial timescales. Quat. Sci. Rev. 237, 106299 (2020).
Di Roberto, A. et al. First marine cryptotephra in Antarctica found in sediments of the western Ross Sea correlates with Englacial tephras and climate records. Sci. Rep. 9, 10628 (2019).
Moles, J. et al. Distribution patterns in Antarctic and Subantarctic echinoderms. Polar Biol. 38, 799–813 (2015).
Angulo-Preckler, C., Tuya, F. & Avila, C. Abundance and size patterns of echinoderms in coastal soft-bottoms at Deception Island (South Shetland Islands, Antarctica). Cont. Shelf Res. 137, 131–141 (2017).
Martín-Ledo, R. & López-González, P. J. Brittle stars from Southern Ocean (Echinodermata: Ophiuroidea). Polar Biol. 37, 73–88 (2013).
Sporta-Caputi, S. et al. Seasonal food web dynamics in the Antarctic Benthos of Tethys Bay (ross sea): Implications for biodiversity persistence under different seasonal sea-ice coverage. Front Mar Sci 2020, 7 (2020).
Marina, T. I. et al. The Food Web of Potter Cove (Antarctica): Complexity, structure and function. Estuar. Coast. Shelf Sci. 200, 141–151 (2018).
Michel, L. N. et al. Increased sea ice cover alters food web structure in East Antarctica. Sci. Rep. 9, 8062 (2019).
Norkko, A. et al. Trophic structure of coastal antarctic food webs associated with changes in sea ice and food supply. Ecology 88, 2810–2820 (2007).
O’Hara, T. D., Hugall, A. F., Thuy, B. & Moussalli, A. Phylogenomic resolution of the class Ophiuroidea unlocks a global microfossil record. Curr. Biol. 24, 1874–1879 (2014).
Thuy, B. & Stohr, S. A new morphological phylogeny of the ophiuroidea (echinodermata) accords with molecular evidence and renders microfossils accessible for cladistics. PLoS One 11, e0156140 (2016).
Thuy, B. & Stohr, S. Lateral arm plate morphology in brittle stars (Echinodermata: Ophiuroidea): New perspective for ophiuroid micropaleontology and classification. Zootaxa 3013, 1–47 (2011).
Clark, M. S. et al. Delayed arm regeneration in the antarctic brittle star Ophionotus victoriae. Aquat. Biol. 1, 45–53 (2007).
Herguera, J. C. & Berger, W. H. Paleoproductivity from benthic foraminifera abundance: Glacial to postglacial change in the west-equatorial Pacific. Geology 19, 1173–1176 (1991).
Anderson, J. B. Ecology and distribution of foraminifera in the Weddel Sea of Antarctica. Micropaleontology 21, 69–96 (1975).
Capotondi, L., Bonomo, S., Budillon, G., Giordano, P. & Langone, L. Living and dead benthic foraminiferal distribution in two areas of the Ross Sea (Antarctica). Rend Lincei Sci. Fis. Nat. 31, 1037–1053 (2020).
Majewski, W. & Anderson, J. B. Holocene foraminiferal assemblages from Firth of Tay, Antarctic Peninsula: Paleoclimate implications. Mar. Micropaleontol. 73, 135–147 (2009).
Majewski, W., Bart, P. J. & McGlannan, A. J. Foraminiferal assemblages from ice-proximal paleo-settings in the Whales Deep Basin, eastern Ross Sea, Antarctica. Palaeogeogr. Palaeoclimatol. Palaeoecol. 493, 64–81 (2018).
Li, B., Yoon, H. & Park, B. Foraminiferal assemblages and CaCO3 dissolution since the last deglaciation in the Maxwell Bay, King George Island, Antarctica. Mar. Geol. 169, 239–257 (2000).
McKnight, D. G. Echinoderms from Cape Hallett, Ross Sea. N. Z. J. Mar. Freshw. Res. 1, 314–323 (1967).
Simpson, G. L. Modelling palaeoecological time series using generalised additive models. Front. Ecol. Evol. 2018, 6 (2018).
Hastie, T. & Tibshirani, R. Generalized additive models: Some applications. J. Am. Stat. Assoc. 82, 371–386 (1987).
R Core Team. R: A Language and Environment for Statistical Computing (2023).
Simon, W. mgcv: GAMs and generalized ridge regression for R. R News 1, 20–25 (2001).
Dunnington, D. W., Libera, N., Spooner, I. S., Kurek, J. & Gagnon, G. A. tidypaleo: Visualizing paleoenvironmental archives using ggplot2. J. Stat. Softw. 101(7), 1–19 (2022).
Hammer, O., Harper, D. T. A. & Ryan, P. D. PAST: Paleontological Statistic Software package for education and data analyses. Palaeontol. Electron. 4, 963 (2001).
Chiantore, M. et al. Sea urchins, sea stars and brittle stars from Terra Nova Bay (Ross Sea, Antarctica). Polar Biol. 29, 467–475 (2005).
Fratt, D. B. & Dearborn, J. H. Feeding biology of the Antarctic brittle star Ophionotus victoriae (Echinodermata: Ophiuroidea). Polar Biol. 3, 127–139 (1984).
Mcclintock, J. B. Trophic biology of Antarctic shallow-water echinoderms. Mar. Ecol. Progress Ser. 111, 191–202 (1994).
Angulo-Preckler, C. et al. Macrobenthic patterns at the shallow marine waters in the caldera of the active volcano of Deception Island, Antarctica. Cont. Shelf. Res. 157, 20–31 (2018).
Grange, L. J., Tyler, P. A., Peck, L. & Cornelius, N. Long-term interannual cycles of the gametogenic ecology of the Antarctic brittle star Ophionotus victoriae. Mar. Ecol. Prog. Ser. 278, 14–155 (2004).
Walker, B. J., Miller, M. F., Bowser, S. S., Furbish, D. J. & Gualda, G. A. R. Dissolution of ophiuroid ossicles on the shallow antarctic shelf: Implications for the fossil record and ocean acidification. Palaios 28, 317–332 (2013).
Hauck, J. et al. Distribution and mineralogy of carbonate sediments on Antarctic shelves. J. Mar. Syst. 90, 77–87 (2012).
Duquette, A., Halanych, K. M., Angus, R. A. & McClintock, J. B. Inter and intraspecific comparisons of the skeletal Mg/Ca ratios of high latitude Antarctic echinoderms. Antarct. Sci. 30, 160–169 (2018).
Ghiold, J. Biological oceanography species distributions of irregular echinoids. Biol. Oceanogr. 6, 79–162 (1989).
Schlüter, N., Wiese, F. & Reich, M. Systematic assessment of the Atelostomata (Spatangoida and Holasteroida; irregular echinoids) based on spine microstructure. Zool. J. Linn. Soc. 175, 510–524 (2015).
Pascal, P. Y., Reynaud, Y., Poulin, E., De Ridder, C. & Saucede, T. Feeding in spatangoids: The case of Abatus cordatus in the Kerguelen Islands (Southern Ocean). Polar Biol. 44, 795–808 (2021).
Peck, V. L., Allen, C. S., Kender, S., McClymont, E. L. & Hodgson, D. A. Oceanographic variability on the West Antarctic Peninsula during the Holocene and the influence of upper circumpolar deep water. Quat. Sci. Rev. 119, 54–65 (2015).
Zhou, Y. et al. Enhanced iceberg discharge in the western North Atlantic during all Heinrich events of the last glaciation. Earth Planet Sci. Lett. 564, 116910 (2021).
Reeh, N., Mayer, C., Miller, H., Thomsen, H. H. & Weidick, A. Present and past climate control on fjord glaciations in Greenland: Implications for IRD-deposition in the sea. Geophys. Res. Lett. 26, 1039–1042 (1999).
Eynaud, F. et al. Position of the Polar Front along the western Iberian margin during key cold episodes of the last 45 ka. Geochem. Geophys. Geosyst. 10, 05 (2009).
Christ, A. J. et al. Late Holocene glacial advance and ice shelf growth in Barilari Bay, Graham Land, West Antarctic Peninsula. Bull. Geol. Soc. Am. 127, 297–315 (2015).
Cornelius, N. & Gooday, A. J. ‘Live’ (stained) deep-sea benthic foraminifera in the western Weddell Sea: Trends in abundance, diversity and taxonomic composition along a depth transect. Deep Sea Res. Part II: Top. Stud. Oceanogr. 51, 1571–1602 (2004).
Gooday, A. J., Bett, B. J., Jones, D. O. B. & Kitazato, H. The influence of productivity on abyssal foraminiferal biodiversity. Mar. Biodivers. 42, 415–431 (2012).
Włodarska-Kowalczuk, M., Pawłowska, J. & Zajaczkowski, M. Do foraminifera mirror diversity and distribution patterns of macrobenthic fauna in an Arctic glacial fjord?. Mar. Micropaleontol. 103, 30–39 (2013).
Buzas, M. A. & Hayek, L.-A.C. On richness and evenness within and between communities. Paleobiology 31, 199–220 (2005).
Zou, Y., Zhao, P. & Axmacher, J. C. Estimating total species richness: Fitting rarefaction by asymptotic approximation. Ecosphere 14, e4363 (2023).
Belt, S. T. et al. Source identification and distribution reveals the potential of the geochemical Antarctic sea ice proxy IPSO25. Nat. Commun. 7, 12655 (2016).
Lüning, S., Gałka, M. & Vahrenholt, F. The medieval climate anomaly in Antarctica. Palaeogeogr. Palaeoclimatol. Palaeoecol. 532, 109251 (2019).
Mezgec, K. et al. Holocene sea ice variability driven by wind and polynya efficiency in the Ross Sea. Nat. Commun. 8, 1334 (2017).
Stenni, B. et al. Antarctic climate variability on regional and continental scales over the last 2000 years. Clim. Past 13, 1609–1634 (2017).
Rhodes, R. H. et al. Little Ice Age climate and oceanic conditions of the Ross Sea, Antarctica from a coastal ice core record. Clim. Past 8, 1223–1238 (2012).
Acknowledgements
This work was funded by Italian MIUR-PNRA program (Ministero dell’Università e della Ricerca—Programma Nazionale di Ricerche in Antartide) in the framework of the following projects: EDISTHO (grant PNRA2018_00010. PI: Karen Gariboldi), TRACERS (Grant PNRA2016-A3/00055. PI: Alessio di Roberto) and HOLOFERNE (PEA2013/AN2.03. PI: Leonardo Langone). The authors are deeply grateful to Alessio Di Roberto and Leonardo Langone for the great effort put in planning and managing TRACERS and HOLOFERNE and in the recovering of core TR17_08PC, the object of this study. We are also very thankful to Patrizia Giordano for the unpayable work in reconstructing the core absolute depths. We also thank Leonardo Langone for the critical review of the manuscript. Finally, the results achieved in this paper were possible thanks to the great help that Carlo Gaetan has provided in suggesting how to deal with GAMs and thanks to his critical review of the work, especially concerning the statistical analysis.
Author information
Authors and Affiliations
Contributions
G.G. wrote the main manuscript text, conducted the main analysis and prepared the figures. B.T. helped for the identification of the echinoderm ossicles. K. G. founding acquisition . All author reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Galli, G., Morigi, C., Thuy, B. et al. Late Holocene echinoderm assemblages can serve as paleoenvironmental tracers in an Antarctic fjord. Sci Rep 14, 15300 (2024). https://doi.org/10.1038/s41598-024-66151-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-66151-5